Community Mitigation Guidelines to Prevent Pandemic Influenza — United States, 2017
Recommendations and Reports / April 21, 2017 / 66(1);1–34
Noreen Qualls, DrPH1; Alexandra Levitt, PhD2; Neha Kanade, MPH1,3; Narue Wright-Jegede, MPH1,4; Stephanie Dopson, ScD5; Matthew Biggerstaff, MPH6; Carrie Reed, DSc6; Amra Uzicanin, MD1 (View author affiliations)
View suggested citationSummary
When a novel influenza A virus with pandemic potential emerges, nonpharmaceutical interventions (NPIs) often are the most readily available interventions to help slow transmission of the virus in communities, which is especially important before a pandemic vaccine becomes widely available. NPIs, also known as community mitigation measures, are actions that persons and communities can take to help slow the spread of respiratory virus infections, including seasonal and pandemic influenza viruses.
These guidelines replace the 2007 Interim Pre-pandemic Planning Guidance: Community Strategy for Pandemic Influenza Mitigation in the United States — Early, Targeted, Layered Use of Nonpharmaceutical Interventions (https://stacks.cdc.gov/view/cdc/11425). Several elements remain unchanged from the 2007 guidance, which described recommended NPIs and the supporting rationale and key concepts for the use of these interventions during influenza pandemics. NPIs can be phased in, or layered, on the basis of pandemic severity and local transmission patterns over time. Categories of NPIs include personal protective measures for everyday use (e.g., voluntary home isolation of ill persons, respiratory etiquette, and hand hygiene); personal protective measures reserved for influenza pandemics (e.g., voluntary home quarantine of exposed household members and use of face masks in community settings when ill); community measures aimed at increasing social distancing (e.g., school closures and dismissals, social distancing in workplaces, and postponing or cancelling mass gatherings); and environmental measures (e.g., routine cleaning of frequently touched surfaces).
Several new elements have been incorporated into the 2017 guidelines. First, to support updated recommendations on the use of NPIs, the latest scientific evidence available since the influenza A (H1N1)pdm09 pandemic has been added. Second, a summary of lessons learned from the 2009 H1N1 pandemic response is presented to underscore the importance of broad and flexible prepandemic planning. Third, a new section on community engagement has been included to highlight that the timely and effective use of NPIs depends on community acceptance and active participation. Fourth, to provide new or updated pandemic assessment and planning tools, the novel influenza virus pandemic intervals tool, the Influenza Risk Assessment Tool, the Pandemic Severity Assessment Framework, and a set of prepandemic planning scenarios are described. Finally, to facilitate implementation of the updated guidelines and to assist states and localities with prepandemic planning and decision-making, this report links to six supplemental prepandemic NPI planning guides for different community settings that are available online (https://www.cdc.gov/nonpharmaceutical-interventions).
Introduction
Nonpharmaceutical interventions (NPIs) are strategies for disease, injury, and exposure control (https://www.cdc.gov/phpr/capabilities/DSLR_capabilities_July.pdf). They include actions that persons and communities can take to help slow the spread of respiratory viruses (e.g., seasonal and pandemic influenza viruses). These actions include personal protective measures for everyday use (e.g., staying home when ill, covering coughs and sneezes, and washing hands often) and communitywide measures reserved for pandemics and aimed at reducing opportunities for exposure (e.g., coordinated closures and dismissals of child care facilities and schools and cancelling mass gatherings). When a novel influenza A virus with pandemic potential emerges, NPIs can be used in conjunction with available pharmaceutical interventions (antiviral medications) to help slow its transmission in communities, especially when a vaccine is not yet widely available. Given current vaccine technology, a pandemic vaccine might not be available for up to 6 months (https://www.fda.gov/%20ForConsumers/ConsumerUpdates/ucm336267.htm). NPIs can be used before a pandemic is declared in areas where a novel influenza A virus is detected and during a pandemic.
These 2017 guidelines provide evidence-based recommendations on the use of NPIs in mitigating the effects of pandemic influenza. These guidelines update and expand the 2007 strategy (https://stacks.cdc.gov/view/cdc/11425).*
Purpose
The purpose of these guidelines is to help state, tribal, local, and territorial health departments with prepandemic planning and decision-making by providing updated recommendations on the use of NPIs. These recommendations have incorporated lessons learned from the federal, state, and local responses to the influenza A (H1N1)pdm09 virus pandemic (hereafter referred to as the 2009 H1N1 pandemic) and findings from research. Communities, families and individuals, employers, and schools can create plans that use these interventions to help slow the spread of a pandemic and prevent disease and death.
Specific goals for implementing NPIs early in a pandemic include slowing acceleration of the number of cases in a community, reducing the peak number of cases during the pandemic and related health care demands on hospitals and infrastructure, and decreasing overall cases and health effects ( Figure 1). When a pandemic begins, public health authorities need to decide on an appropriate set of NPIs for implementation and to reiterate the importance of personal protective measures for everyday use (e.g., voluntary home isolation of ill persons [staying home when ill], respiratory etiquette, and hand hygiene) and environmental cleaning measures (e.g., routine cleaning of frequently touched surfaces), which are recommended at all times for prevention of respiratory illnesses ( Table 1). Personal protective measures reserved for pandemics (e.g., voluntary home quarantine of exposed household members [staying home when a household member is ill] and use of face masks by ill persons) also might be recommended (Table 1). A more difficult decision is how and when to implement community-level NPIs that might be warranted but are more disruptive (e.g., temporary school closures and dismissals, social distancing in workplaces and the community, and cancellation of mass gatherings) (Table 1). These decisions are made by state and local officials on the basis of conditions in the applicable jurisdictions, with guidance from CDC (according to pandemic severity and potential efficacy) and governing authorities (1). Prepandemic planning, along with community engagement, is an essential component of these decisions ( Table 2).
The decision regarding whether and when to recommend additional NPIs is another component ( Table 3). State and local public health departments might use certain influenza surveillance indicators to help decide when to consider implementing NPIs such as school closures and dismissals and other social distancing measures in schools, workplaces, and public settings during an influenza pandemic. The choice of influenza surveillance indicators might differ among states and localities, depending on the availability and capacity of their public health resources. Examples of possible influenza surveillance indicators include additional patient visits to health care providers for influenza-like illness (ILI) and increased geographic spread of influenza within a state. Indicators for school closures and dismissals might include increased school absenteeism rates or the earliest laboratory-confirmed influenza cases among students, teachers, or staff members. Indicators that might help confirm that NPI implementation should continue include increased influenza-associated hospitalizations or increases in adult or pediatric deaths attributed to influenza. Additional information about NPI prepandemic planning is available (supplementary Chapter 1 https://stacks.cdc.gov/view/cdc/44313).
Background
An influenza pandemic occurs when a novel virus emerges for which the majority of the population has little or no immunity. Influenza pandemics are facilitated by sustained human-to-human transmission, and the infection spreads worldwide over a relatively short period (2). The first influenza pandemic of the 21st century began in 2009, 2 years after the 2007 strategy for prepandemic planning was published. Lessons learned during the response to the 2009 H1N1 pandemic underscored the importance of a flexible approach to the use of NPIs, particularly during the early stages of a pandemic, and led to the development of new tools for assessing pandemic severity and prepandemic planning ( Box 1).
Lessons Learned from the 2009 H1N1 Pandemic Response
The 2009 H1N1 pandemic was a reminder to be prepared for the unpredictable nature of pandemics. Knowing in advance which subtype of pandemic virus will emerge is impossible, as is where and when it will emerge, how quickly the virus will spread, how severe the illness will be, and who will be the most affected. Because of this unpredictability, prepandemic planning must be broad and flexible.
The 2007 strategy for prepandemic planning was developed with the assumption that the next influenza pandemic would be severe, like the 1957 pandemic, which was characterized by high transmissibility and medium clinical severity. When the 2007 strategy was developed, the primary concern was that a pandemic virus might evolve from the highly pathogenic avian influenza A (H5N1) virus, a virus that reemerged in Asia in 2003 in domestic poultry and spread to Africa, the Middle East, and Europe among poultry, with sporadic zoonotic transmission (37). Moreover, CDC thought that this virus would most likely emerge overseas, providing the United States with time to prepare for a domestic response, including making use of prepandemic H5N1 vaccine in CDC’s Strategic National Stockpile. Instead, the 2009 pandemic influenza A virus turned out to be a novel H1N1 virus that appears to have emerged in southern Mexico and was first identified in two persons in California (13). Although the 2009 H1N1 pandemic in the United States was moderate in terms of overall morbidity and mortality among the U.S. general population, severe outcomes from H1N1pdm09 virus infection were more common among children, young adults, and specific groups at risk for serious complications (e.g., pregnant women) than among older adults (Box 1).
Although the emergence of the H1N1pdm09 virus prompted development of pandemic vaccines, a pandemic vaccine was not available until October 2009, 6 months after the initial report that identified the pandemic virus. In addition, another 2 months were required (December 2009) for sufficient stocks to be manufactured, distributed, and available to vaccinate several population groups, including school-aged children and persons living with or caring for infants aged <6 months, as recommended by the Advisory Committee on Immunization Practices (ACIP).† Even though work is ongoing to accelerate the pace of development, distribution, and administration of a vaccine during future pandemics, this experience reaffirmed the importance of the use of NPIs in the early stages of a pandemic before a well-matched vaccine is widely available (i.e., vaccines produced using a virus that is very similar to the circulating virus).
Another lesson learned about NPI implementation during the 2009 H1N1 pandemic was that rapidly changing guidance can create confusion and difficulties during implementation (Box 1) (30,31). Nevertheless, field studies found that school-related NPIs, including school closures recommended to mitigate the impact of the 2009 H1N1 pandemic during spring 2009, were considered acceptable and feasible for most parents and caregivers, even when parents had to miss work and in the absence of free or reduced-cost school lunches for students (28,38–41). Other interventions that reduced the spread of H1N1pdm09 virus in some communities included hand hygiene (42), regularly scheduled school summer breaks (19), and social distancing measures, such as cancelling mass gatherings and closing public places (22).
Community Engagement
The 2009 H1N1 pandemic underscored that effective prepandemic planning requires the involvement of public health and local leaders, employers, organizations, and stakeholders and is essential to ensure timely and effective use of NPIs to limit disease spread during a pandemic ( Box 2). Effective use of NPIs depends on the acceptance and participation of individual persons who implement personal protective measures and of communities that implement communitywide measures such as temporary school closures (https://www.cdc.gov/phpr/capabilities/DSLR_capabilities_July.pdf).
The 2007 guidance took into account the results of a 2006 opinion poll conducted with a representative national sample of 1,697 adults aged ≥18 years. The results indicated that when faced with an outbreak of pandemic influenza, the majority of persons in the United States would be willing to make major changes in their lives and cooperate with public health recommendations on the use of NPIs (http://archive.sph.harvard.edu/press-releases/2006-releases/press10262006.html). Findings were similar in a follow-up study during the 2009–2010 H1N1 pandemic (Box 1) (https://www.hsph.harvard.edu/horp/project-on-the-public-response-to-h1n1).
For example, in 2006, 85% of the respondents said that they and all members of their household would stay home for 7–10 days if another household member were ill with pandemic influenza. The H1N1 opinion polls also identified barriers to implementation of NPIs among persons and communities (e.g., the ability to stay home when ill, job security, and income protection) (https://www.hsph.harvard.edu/horp/project-on-the-public-response-to-h1n1). States and localities could establish local planning councils or hold public engagement meetings that address these and other issues related to public health preparedness, pandemic education, and planning. States and local communities also can draw on planning guidance provided in the CDC Public Health Preparedness Capabilities: National Standards for State and Local Planning, which lists NPIs as one of 15 capabilities (https://www.cdc.gov/phpr/capabilities/DSLR_capabilities_July.pdf). Additional information about pandemic influenza and NPI community engagement is available (supplementary Chapter 1 https://stacks.cdc.gov/view/cdc/44313).
New Tools for Prepandemic Planning and Pandemic Assessment
Novel Influenza Virus Pandemic Intervals
In 2014, CDC updated its 2008 guidance on pandemic intervals to include six intervals that describe influenza pandemic progression in a way that supports flexible prepandemic preparedness and response. The intervals include 1) investigation of novel influenza cases, 2) recognition of potential for ongoing transmission, 3) initiation, 4) acceleration, 5) deceleration of the pandemic wave, and 6) preparation for a future pandemic wave (43). These intervals can be used during prepandemic planning and can serve as a platform for public health decision-making and actions during the beginning of a potential influenza pandemic. Each interval is associated with particular response activities, including implementation of select NPIs during the initiation and acceleration intervals and coordinated discontinuation of select community-level NPIs reserved for pandemics during the deceleration interval ( Figure 2) ( Table 4). Although the six-interval framework describes the sequence of pandemic disease evolution over time, the framework does not characterize the transmissibility of the virus or the clinical severity of the outbreak. Therefore, CDC has developed additional tools for pandemic planning and response, including the Influenza Risk Assessment Tool (supplementary Chapter 2 https://stacks.cdc.gov/view/cdc/44313); https://www.cdc.gov/flu/pandemic-resources/tools/risk-assessment.htm) and the Pandemic Severity Assessment Framework (PSAF). Additional information about the pandemic intervals is available (supplementary Chapter 2 https://stacks.cdc.gov/view/cdc/44313).
Pandemic Severity Assessment Framework
An influenza pandemic can range from mild to extremely severe in terms of clinical severity and transmission rate. When a pandemic emerges, public health authorities should assess its projected impact and recommend rapid action to reduce virus transmission, protect populations at high risk for complications, and minimize societal disruption. As observed during the 2009 H1N1 pandemic response, attack rates and case-fatality ratios can be difficult to measure early in a pandemic because of variations in care-seeking behavior and testing practices; not everyone seeks care for their illness, and not everyone is tested and receives a diagnosis of pandemic influenza. As a result, severe cases might be more likely to be reported, resulting in an overestimate of the case-hospitalization or case-fatality ratio. Tools for prepandemic planning have been updated and augmented based on that experience, and the Pandemic Severity Index in the 2007 guidance has been replaced with PSAF. PSAF uses multiple clinical and epidemiologic indicators to provide a more comprehensive assessment of the transmissibility and clinical severity of an emerging pandemic. Whereas the Pandemic Severity Index was based on the assumption that a future pandemic would cause an illness rate of 30% in the U.S. population and relied on an assessment of case-fatality ratios to determine severity of an evolving pandemic, PSAF incorporates multiple measures of clinical severity (e.g., case-fatality ratios, case-hospitalization ratios, and deaths-hospitalizations ratios) and viral transmissibility (e.g., secondary household attack rates, school attack rates, workplace attack rates, community attack rates, or all of these, as well as rates of emergency department and outpatient visits for ILI) (44).
When a pandemic begins, in the United States or anywhere in the world, CDC makes an initial assessment of viral transmissibility and clinical severity on the basis of these multiple PSAF measures (Table 5) (44). On the basis of the initial assessment, CDC recommends that affected U.S. jurisdictions respond (and other jurisdictions prepare to respond). Although data are limited during the initial 3–4 weeks after the emergence of a pandemic virus, these early data are compiled into a broad, preliminary assessment. CDC uses PSAF scores of viral transmissibility and clinical severity to place the pandemic within one of four assessment quadrants ( Figure 3). Depending on the surveillance capacity in the location where the novel virus emerges and first spreads, 4–8 weeks or longer might be required to accrue sufficient data for a refined assessment of an evolving pandemic. Once data are available, the refined assessment is used to more precisely characterize the clinical severity and transmissibility of the pandemic virus ( Figure 4) ( Table 6). These initial and refined assessments of pandemic severity are used, in coordination with state and local public health partners, to guide the use of NPI measures. Additional information about PSAF is available (supplementary Chapter 2 https://stacks.cdc.gov/view/cdc/44313).
Methods
Guidelines Development Process
This 2017 update consists of three separate documents: this report and two supplementary documents (https://stacks.cdc.gov/view/cdc/44313 and https://stacks.cdc.gov/view/cdc/44314). This report provides a brief introduction to pandemic influenza and NPIs; describes the 2007 strategy and the purpose of the updates, particularly after the 2009 H1N1 pandemic; outlines the methods used to develop this update and describe the evidence considered for NPI use during an influenza pandemic; presents CDC’s NPI recommendations; and discusses key areas for further NPI research. The two supplementary documents contain more specific and detailed information about pandemic influenza and NPIs. One document (Technical Report 1 https://stacks.cdc.gov/view/cdc/44313) is divided into chapters and provides an introduction to and overview of NPIs, a description of the new tools developed for pandemic influenza planning and assessment, and a toolbox describing the NPI evidence base, implementation issues, and research gaps. The second document (Technical Report 2 https://stacks.cdc.gov/view/cdc/44314) consists of several appendices that provide a glossary of terms, a detailed description of the methods used for developing the NPI recommendations, a comprehensive summary table of the NPI body of evidence, and a list of tools and resources for pandemic influenza planning and preparedness.
This 2017 update was developed through collaboration involving input from several sources, including peer-reviewed scientific literature, current research, CDC subject-matter experts, and external stakeholders (e.g., federal agencies, public health officials, and business and education partners). Development of these updated guidelines involved participation by multiple CDC groups (e.g., the Community Mitigation Guidelines Work Group and the coordination, abstraction, and consultation teams), as well as a group of external stakeholders who reviewed a document, summarizing the overall direction and key principles and concepts of the guidelines. Input from the work group members, subject-matter experts, and stakeholders was considered and incorporated during the creation of the 2017 planning guidelines. The guidelines were developed during October 2011–October 2016 ( Table 7). The complete list of contributors and their roles in the process are available (supplementary Appendix 2 https://stacks.cdc.gov/view/cdc/44314).
Use of NPIs During Influenza Pandemics
Ten years ago, when the 2007 strategy was being developed, the evidence for the use of NPIs during influenza pandemics was limited, consisting primarily of historical analyses and contemporary observations rather than controlled scientific studies (45,46). These analyses and observations were supplemented by modeling studies that used historical data to evaluate NPI use in U.S. cities during the 1918 pandemic (47,48) or that simulated pandemic scenarios as they might occur in the future (49–51). The simulations, like the historical analyses, generally supported the effectiveness of early, targeted, and phased-in (layered) use of multiple NPIs§ in preventing spread of disease, especially when used in combination with antiviral medications (46,49). This conclusion seemed plausible, confirming the presumption that individual, partially effective NPIs act in complementary ways to decrease various factors that facilitate the spread of influenza under different circumstances and settings (52). However, the NPI modeling studies had substantial limitations, including lack of data supporting assumptions about the effectiveness of individual NPIs, economic and social costs of NPIs, and likely rates of compliance (46,49,53).
In 2016, the evidence supporting the effectiveness of NPIs, both when used alone and in combination, was more substantial and included controlled studies evaluating different NPIs. New modeling studies based on data collected during the 2009 H1N1 pandemic response also became available. This update is based on approximately 191 journal articles written in English and published from 1990 through September 2016 that focused on personal protective measures in general; school closure effectiveness and unintended consequences; school absenteeism; spread of disease in child care facilities, colleges, and universities; impact of mass gatherings; and role and impact of NPIs in non–health care workplace settings. These articles were reviewed, abstracted, and synthesized. To assess the strength of the evidence, a five-step NPI rating scheme process was developed by adapting and applying the approach of the Guide to Community Preventive Services (The Community Guide) (https://www.thecommunityguide.org). Additional information about the NPI rating scheme process is available (supplementary Appendices 3 and 4 https://stacks.cdc.gov/view/cdc/44314).
The selected articles were organized into three groups: 1) personal NPIs (personal protective measures for everyday use and personal protective measures reserved for influenza pandemics); 2) community NPIs (social distancing measures and school closures and dismissals); and 3) environmental NPIs (surface cleaning measures) ( Table 8). Key steps included selecting the relevant literature, abstracting and synthesizing the evidence, and assessing the evidence quality (both individual study quality and quality of the body of evidence). A recommendation was formulated based on the evidence of effectiveness for each NPI. The strength of NPI recommendations took into consideration the effectiveness of the intervention, the ease of implementation (including unwanted consequences), and the importance of the intervention as a public health strategy. Additional information about the NPI evidence base is available (supplementary Chapter 3 https://stacks.cdc.gov/view/cdc/44313 and supplementary Appendix 5 https://stacks.cdc.gov/view/cdc/44314).
Recommendations on the Use of Personal, Community, and Environmental NPIs
NPIs routinely recommended for prevention of respiratory virus transmission, such as seasonal influenza, include personal protective measures for everyday use (i.e., voluntary home isolation of ill persons, respiratory etiquette, and hand hygiene) and environmental surface cleaning measures (i.e., routine cleaning of frequently touched surfaces and objects). During an influenza pandemic, these NPIs are recommended regardless of the pandemic severity level. Additional personal and community NPIs also might be recommended. Personal protective measures reserved for pandemics include voluntary home quarantine of exposed household members and use of face masks in community settings when ill. Community NPIs might include temporary closures or dismissals of child care facilities and schools with students in grades kindergarten through 12 (K–12), as well as other social distancing measures that increase the physical space between people (e.g., workplace measures such as replacing in-person meetings with teleconferences or modifying, postponing, or cancelling mass gatherings) ( Figure 5) (Table 1). Local decisions about NPI selection and timing involve consideration of overall pandemic severity and local conditions (1) and require flexibility and possible modifications as the pandemic progresses and new information becomes available.
Updated recommendations on the use of NPIs to help slow the spread and decrease the impact of an influenza pandemic are provided, as is information on the rationale for using each NPI as part of a comprehensive public health strategy for pandemic response and the appropriate settings and use for each NPI according to the severity of the pandemic ( Table 9).¶ The recommendations that follow are considered an update to the existing recommendations in the 2007 guidance because the same set of NPIs has been maintained and recommended for use early in a pandemic. However, the difference between the guidance issued in 2007 and in 2017 is the clear delineation of NPIs into two categories: 1) NPIs recommended at all times and 2) NPIs recommended for use only during pandemics (based on the level of pandemic severity and local conditions). The 2017 update also provides additional evidence to support the NPI recommendations.
Personal NPIs
NPIs that can be implemented by individual persons include the following:
- Personal protective measures for everyday use: These include voluntary home isolation of ill persons, respiratory etiquette, and hand hygiene.
- Personal protective measures reserved for pandemics: These include voluntary home quarantine of exposed household members and use of face masks in community settings when ill.
Personal Protective Measures for Everyday Use
Personal protective measures are preventive actions that can be used daily to slow the spread of respiratory viruses (https://www.cdc.gov/nonpharmaceutical-interventions/personal/index.html; supplementary Chapter 3 https://stacks.cdc.gov/view/cdc/44313). These measures include the following:
- Voluntary home isolation (i.e., staying home when ill or self-isolation): Persons with influenza stay home for at least 24 hours after a fever or signs of a fever (chills, sweating, and feeling warm or flushed)** are gone (https://www.cdc.gov/flu/protect/preventing.htm), except to obtain medical care or other necessities.†† To ensure that the fever is gone, patients’ temperature should be measured in the absence of medication that lowers fever (e.g., acetaminophen or ibuprofen). In addition to fever, common influenza symptoms include cough or chest discomfort, muscle or body aches, headache, and fatigue. Persons also might experience sneezing, a runny or stuffy nose, sore throat, vomiting, and diarrhea (https://www.cdc.gov/flu/consumer/symptoms.htm).
- Respiratory etiquette: Persons cover coughs and sneezes, preferably with a tissue, and then dispose of tissues and disinfect hands immediately after a cough or sneeze, or (if a tissue is not available) cough or sneeze into a shirt sleeve. Touching the eyes, nose, and mouth should be avoided to help slow the spread of germs (https://www.cdc.gov/flu/protect/covercough.htm).
- Hand hygiene: Persons perform regular and thorough hand washing with soap and water (or use alcohol-based hand sanitizers containing at least 60% ethanol or isopropanol when soap and water are not available).
Rationale for use as a public health strategy. Most persons infected with an influenza virus might become infectious 1 day before the onset of symptoms and remain infectious up to 5–7 days after becoming ill (54,55). However, studies found that infants and immunocompromised persons might shed influenza viruses for prolonged periods (up to 21 days and a mean of 19 days, respectively) (56,57). The effectiveness of personal protective measures depends on their ability to interrupt virus transmission from one person to another. Voluntary home isolation, which is a form of patient isolation, prevents an ill person from infecting other people outside of their household.§§ Respiratory etiquette reduces the dispersion of droplets contaminated with influenza virus being propelled through the air by coughing or sneezing. Hand hygiene reduces the transmission of influenza viruses that occurs when one person touches another (e.g., with a contaminated hand). Contamination also can occur through self-inoculation via fomite transmission (indirect contact transmission) when persons touch a contaminated surface and then touch their nose with a contaminated hand. A study conducted in households in Bangkok, Thailand, found that increased handwashing reduced surface contamination with influenza virus, which lowered the potential for self-inoculation via fomite transmission (58). Additional studies found that influenza viruses can remain viable on the human hand for roughly 3–5 minutes (59) and that influenza viruses can remain on fingers for 30 minutes after contamination (60).
Settings and use. Voluntary home isolation involves persons remaining at home when ill with influenza. Respiratory etiquette and hand hygiene are recommended in homes and in all other community settings, including schools and workplaces. All three personal protective measures are considered everyday preventive actions that should be implemented year-round but that are especially important during annual influenza seasons and influenza pandemics ( Table 10). Use of these personal protective measures might result in some secondary (unintended or unwanted) consequences (e.g., concerns about job security for ill persons who lack paid sick leave or skin irritations due to frequent hand washing).
Personal Protective Measures Reserved for Pandemics
Voluntary home isolation, respiratory etiquette, and hand hygiene are recommended during both annual influenza seasons and influenza pandemics. Additional personal protective measures that might be recommended during pandemics include voluntary home quarantine of exposed household members and the use of face masks in community settings when ill. These measures might contribute to reductions in transmission of pandemic influenza viruses when the level of pandemic severity and local conditions warrant their use (supplementary Chapter 3https://stacks.cdc.gov/view/cdc/44313).
Voluntary Home Quarantine
Voluntary home quarantine of non-ill household members of persons with influenza (also called self-quarantine or household quarantine) helps prevent disease spread from households to schools, workplaces, and other households because those household members have been exposed to the influenza virus. Exposed household members of symptomatic persons (with confirmed or probable pandemic influenza) should stay home for up to 3 days (the estimated incubation period for seasonal influenza) (61) starting from their initial contact with the ill person. If they then become ill, they should practice voluntary home isolation (i.e., they should remain at home until recovered as discussed previously; https://www.cdc.gov/quarantine/index.html). For certain exposed household members (e.g., those at high risk for influenza complications or with severe immune deficiencies), guidelines should be consulted regarding the prophylactic use of antiviral medications (https://www.cdc.gov/flu/professionals/antivirals/index.htm).
Rationale for use as a public health strategy. Voluntary home quarantine might help slow a pandemic by reducing community transmission from households with a person who has influenza because the exposed household members are at increased risk for infection. Furthermore, certain infected (but not yet symptomatic) household members could begin shedding influenza virus at least a day before exhibiting symptoms and could infect friends, neighbors, and others in the community (e.g., at school or work) before becoming symptomatic. Therefore, all members of a household with a symptomatic person (with confirmed or probable pandemic influenza) might be asked to stay home for a specified period of time (up to 3 days) to assess for early signs and symptoms of pandemic influenza virus infection. If other household members become ill during this period, then the time for voluntary home quarantine might need to be extended for another incubation period. The evidence for voluntary home quarantine, particularly when used in combination with other NPIs, includes a systematic literature review, historical analyses of the 1918 pandemic, and mathematical modeling studies (supplementary Chapter 3 https://stacks.cdc.gov/view/cdc/44313 and supplementary Appendix 5 https://stacks.cdc.gov/view/cdc/44314).
Settings and use. Voluntary home quarantine of exposed household members might be recommended during severe, very severe, or extreme influenza pandemics (Table 10) to help reduce the chance of transmitting the virus to others outside of the household. Advance planning is needed to minimize potential secondary consequences for persons who have special cultural, economic, legal, mental, physical, or social status needs (e.g., older adults who depend on necessary community-based services such as home-delivered meals and transportation to health care services). Other secondary consequences might include missed work and loss of income for persons whose employers do not have paid sick leave policies that include home quarantine during pandemics.
Use of Face Masks in Community Settings
Face masks (disposable surgical, medical, or dental procedure masks) are widely used by health care workers to prevent respiratory infections both in health care workers and patients. They also might be worn by ill persons during severe, very severe, or extreme pandemics to prevent spread of influenza to household members and others in the community. However, little evidence supports the use of face masks by well persons in community settings, although some trials conducted during the 2009 H1N1 pandemic found that early combined use of face masks and other NPIs (such as hand hygiene) might be effective (supplementary Chapter 3 https://stacks.cdc.gov/view/cdc/44313).
Rationale for use as a public health strategy. Face masks provide a physical barrier that prevents the transmission of influenza viruses from an ill person to a well person by blocking large-particle respiratory droplets propelled by coughing or sneezing. Face mask use by well persons is not routinely needed in most situations to prevent acquiring the influenza virus. However, use of face masks by well persons might be beneficial in certain situations (e.g., when persons at high risk for influenza complications cannot avoid crowded settings or parents are caring for ill children at home). Face mask use by well persons also might reduce self-inoculation (e.g., touching the nose with the hand after touching a contaminated surface).
Settings and use. Disposable surgical, medical, and dental procedure masks are used widely in health care settings to prevent exposure to respiratory infections. Face masks have few secondary consequences (e.g., discomfort or difficulty breathing) when worn properly and consistently, and face masks sized for children are available. (Additional information about face masks is available at https://www.fda.gov/medicaldevices/productsandmedicalprocedures/generalhospitaldevicesandsupplies/personalprotectiveequipment/ucm055977.htm and https://www.osha.gov/Publications/respirators-vs-surgicalmasks-factsheet.html.)
Community NPIs
NPIs that can be implemented by communities include the following:
- School closures and dismissals: These include temporary closures and dismissals of child care facilities, K–12 schools, and institutions of higher education.
- Social distancing measures: These include measures for schools, workplaces, and mass gatherings.
School Closures and Dismissals
In the event of a pandemic, state and local public health authorities play an important role in protecting the school community and should establish and maintain partnerships with district and school leaders, school emergency operations planning teams, and local municipality leaders (e.g., mayors). Public health authorities are a credible source of information, have multiple (often free) resources available for information awareness campaigns, and provide guidance for increasing school response measures. Depending on the severity of the pandemic, these measures might range from everyday preventive actions to preemptive, coordinated school closures and dismissals. A school closure means closing a school and sending all the students and staff members home, whereas during a school dismissal, a school might stay open for staff members while the children stay home. Preemptive school dismissals can be used to disrupt transmission of influenza before many students and staff members become ill. Coordinated dismissals refer to the simultaneous or sequential closing of schools in a jurisdiction. Thus, preemptive, coordinated school closures and dismissals can be used early during an influenza pandemic to prevent virus transmission in schools and surrounding communities by reducing close contact among the following groups (supplementary Chapter 3 https://stacks.cdc.gov/view/cdc/44313):
- Children in child care centers and preschools
- School-aged children and teens in K–12 schools
- Young adults in institutions of higher education
During a dismissal, the school facilities are kept open, which allows teachers to develop and deliver lessons and materials, thus maintaining continuity of teaching and learning, and allows other staff members to continue to provide services and help with additional response efforts. School closures and dismissals might be coupled with social distancing measures (e.g., cancelling sporting events and other mass gatherings) to reduce out-of-school social contact among children when schools are closed.
Rationale for use as a public health strategy. Preventing the spread of disease in educational settings among children and young adults reduces the risk for infection for these age groups and slows virus transmission in the community. Components of the strategy might include preemptive, coordinated school closures and dismissals implemented during the earliest stages of a pandemic, before many students and staff members become ill. Preemptive, coordinated dismissals can be implemented by the following facilities for the following reasons:
- Child care facilities and K–12 schools
- Children have higher influenza attack rates than adults (62) and are infectious for a longer period than adults (63,64).
- Influenza transmission is common in schools and contributes to school absenteeism and parental absenteeism from work (65,66).
- The presence of school-aged children in a household is a risk factor for influenza virus infection in families (62,65,67).
- Social contact and mixing patterns among school-aged children differ substantially depending on the grade and school level, during various periods of the school day, between weekdays and weekends, and between regular school terms and holiday breaks (68–71). Physical floor plans and intergrade activities (e.g., cafeteria size and lunch breaks) also can affect in-school social mixing (68).
- Schoolchildren can introduce the influenza virus into a community, leading to increased rates of illness among their household or community contacts (72–74).
- Institutions of higher education
- Influenza outbreaks on college and university campuses typically have high attack rates (44%–73%) (75–78) and cause substantial morbidity (79,80). For example, during the 2009 H1N1 pandemic, influenza spread rapidly through a university campus within 2 weeks (81); on another residential campus, one infected freshman initiated an outbreak that resulted in 226 laboratory-confirmed cases. Freshmen were the main facilitators of the spread of the H1N1pdm09 virus because of their higher number and frequency of social contacts (82).
- Influenza is more prevalent among residential students at boarding schools and colleges than among nonresidential students (78,83).
- ILIs are common among college and university students and are associated with increased health care use, decreased health status, and impaired school performance (84).
Implementation of preemptive, coordinated school closures and dismissals during an evolving influenza pandemic might have one or more of the following three public health objectives***:
- Objective 1: To gain time for an initial assessment of transmissibility and clinical severity of the pandemic virus in the very early stage of its circulation in humans (closures for up to 2 weeks)
- Objective 2: To slow down the spread of the pandemic virus in areas that are beginning to experience local outbreaks and thereby allow time for the local health care system to prepare additional resources for responding to increased demand for health care services (closures up to 6 weeks)
- Objective 3: To allow time for pandemic vaccine production and distribution (closures up to 6 months)
Two other types of school closures and dismissals might be implemented during a pandemic for public health or institutional reasons. These interventions do not slow disease spread in the community; therefore, they are not considered NPIs. They include the following:
- Selective school closures and dismissals: These might be implemented by schools that serve students at high risk for complications from infection with influenza,††† especially when transmission rates are high. For example, a school that serves children with certain medical conditions or pregnant teens might decide to close while other schools in the area remain open. In addition, some communities or early childhood programs might consider closing child care facilities to help decrease the spread of influenza among children aged <5 years. Selective dismissals are intended to protect persons at high risk for influenza rather than to help reduce virus transmission within the community.
- Reactive school closures and dismissals: These might be implemented when many students and staff members are ill and not attending school or when many students and staff members are arriving at school ill and being sent home. For example, a child care center might close because it is unable to operate under these conditions. Reactive dismissals, which might occur during outbreaks of seasonal influenza (85) and during pandemics (15), are unlikely to affect virus transmission because they typically take place after considerable, if not widespread, transmission has already occurred in the community. For example, a 4-day reactive closure in a western Kentucky school district did not reduce ILI transmission in the rural community (86). Similarly, closing 559 Michigan schools at least once during the fall wave (i.e., second wave) of the 2009 H1N1 pandemic had little effect on community levels of ILI (87).
For more information about preparing for influenza and the different types of dismissals, see CDC websites regarding 1) child care facilities (https://www.cdc.gov/h1n1flu/childcare/toolkit/pdf/childcare_toolkit.pdf), 2) K–12 schools (https://www.cdc.gov/h1n1flu/schools/toolkit/pdf/schoolflutoolkit.pdf), and 3) institutions of higher education (https://www.cdc.gov/h1n1flu/institutions/toolkit/pdf/IHE_toolkit.pdf).
Settings and use. Preemptive, coordinated school closures and dismissals might be implemented at child care facilities, K–12 schools, and institutions of higher education. They are most likely to be implemented when an influenza pandemic is severe, very severe, or extreme (Table 10). Secondary consequences include missed work and loss of income for parents who stay home from work to care for their children and missed opportunities to vaccinate school-aged children rapidly unless other mechanisms are considered.
Social Distancing Measures for Schools, Workplaces, and Mass Gatherings
Social distancing measures can reduce virus transmission by decreasing the frequency and duration of social contact among persons of all ages. These measures are common-sense approaches to limiting face-to-face contact, which reduces person-to-person transmission.
Rationale for use as a public health strategy. Social distancing measures that reduce opportunities for person-to-person virus transmission can help delay the spread and slow the exponential growth of a pandemic. The optimal strategy is to implement these measures simultaneously in places where persons gather. Although direct evidence is limited for the effectiveness of these measures, components of the strategy might include reducing social contacts at the following places:
- Schools: Children have higher influenza attack rates than adults, and influenza transmission is common in schools.
- Workplaces: More than half of all U.S. adults participate in the U.S workforce,§§§ and workers often share office space and equipment and have frequent face-to-face contact. Influenza attack rates in working-age adults (aged 18–64 years) might be as high as 15.5% during a single influenza season (88).
- Mass gatherings: Group events such as concerts, festivals, and sporting events bring people into close contact for extended periods (89–92). A systematic literature review of respiratory disease outbreaks related to mass gatherings in the United States during 2005–2014 indicated that 40 of 72 different outbreaks were associated with state or county agriculture fairs and (zoonotic) transmission of influenza A H3N2v, and 25 outbreaks were associated with residential youth summer camps and person-to-person transmission of influenza A H1N1 (93). An infected traveler attending a mass gathering might introduce influenza to a previously unaffected area, and a person who becomes infected at the event can further spread the infection after returning home (89,90,92,94–96). Even when a circulating virus has a relatively low basic reproductive rate (R0), intensely crowded settings might lead to high secondary attack rates (92). For example, during the 2013 Hajj (Islamic pilgrimage to Mecca) in Saudi Arabia, influenza A/H1N1 virus was found in only two Indonesians on arrival but spread to 25 persons from Africa, Central Asia, and Southeast Asia after the Hajj because of the extremely crowded conditions when performing rituals (97).
Multiple social distancing measures can be implemented simultaneously. Although there is limited empirical evidence supporting the effectiveness of implementing any individual measure alone (other than school closures and dismissals), the evidence for implementing multiple social distancing measures in combination with other NPIs includes systematic literature reviews, historical analyses of the 1918 pandemic, and mathematical modeling studies (supplementary Chapter 3 https://stacks.cdc.gov/view/cdc/44313 and supplementary Appendix 5 https://stacks.cdc.gov/view/cdc/44314).
Settings and use. Social distancing measures can be implemented in a range of community settings, including educational facilities, workplaces, and public places where people gather (e.g., parks, religious institutions, theaters, and sports arenas). The choice of social distancing measure depends on the severity of the pandemic (Table 10). Certain measures might be implemented with few secondary consequences (e.g., increased use of e-mail and teleconferences in some workplaces), whereas others might require advance planning (e.g., modification of mass gatherings). Examples of practical measures that might reduce face-to-face contact in community settings include the following:
- If schools remain open during a pandemic, divide school classes into smaller groups of students and rearrange desks so students are spaced at least 3 feet (98) from each other in a classroom.
- Offer telecommuting and replace in-person meetings in the workplace with video or telephone conferences.
- Modify, postpone, or cancel mass gatherings.
Environmental NPIs: Environmental Surface Cleaning Measures
Environmental surface cleaning measures can help eliminate influenza viruses from frequently touched surfaces and objects, including tables, door knobs, toys, desks, and computer keyboards. These measures involve cleaning surfaces with detergent-based cleaners or disinfectants that have been registered with the Environmental Protection Agency.¶¶¶
Rationale for use as a public health strategy. Although the percentage of influenza cases involving contact transmission (i.e., hand transfer of virus from contaminated objects to the eyes, nose, or mouth) is unknown, this mode of transmission is a recognized route of virus spread (99). The routine use of cleaning measures that eliminate viruses from contaminated surfaces might reduce the spread of influenza viruses (supplementary Chapter 3 https://stacks.cdc.gov/view/cdc/44313).
Settings and use. Environmental surface cleaning measures are recommended for frequently touched surfaces and objects in homes, child care facilities, schools, workplaces, and other places where persons gather. These measures can be used for prevention of seasonal influenza and in all pandemic severity scenarios (Table 10). Use of these measures might result in some secondary consequences (e.g., failing to read instruction labels before applying disinfectants to ensure that they are safe and appropriate to use or cleaning with poor ventilation during the application process).
Discussion
This report expands the NPI guidance presented in the 2007 report by providing evidence-based recommendations on the use of the same set of NPIs. These NPIs include personal protective measures for everyday use and for use during a pandemic, community measures (school closures and dismissals and social distancing), and environmental surface cleaning measures.
Key Concepts Maintained from 2007 Guidance
The rationale for and key concepts regarding the use of NPIs during influenza pandemics first presented in the 2007 guidance remain unchanged. Because production of a pandemic vaccine can take up to 6 months and antiviral medications might be prioritized for treatment, NPIs are likely to be the only prevention tools available early in a pandemic. Therefore, they are critical to slowing the spread of the pandemic influenza virus while a pandemic vaccine is under development.
Like the 2007 strategy, this 2017 update affirms the importance of prepandemic planning and preparedness for use of NPIs during a pandemic response and recommends the early, targeted, and simultaneous implementation of multiple NPIs to decrease influenza virus transmission. Although community-level NPIs can help slow virus transmission, as supported by historical information (100), empirical observations (101), and mathematical modeling (102), these measures are likely to cause unwanted consequences by introducing new norms for social behavior (e.g., adopting precautionary health-protective behaviors such as limiting face-to-face contact with family and friends, only shopping for essential items, avoiding places where people congregate, or not using public transportation) (103), interrupting routine societal functions, and entailing additional costs. If an evolving influenza pandemic is characterized by high clinical severity, the benefits of deploying NPIs, including those with greater potential for secondary consequences, are likely to outweigh potential harms. The more difficult decision is determining how and when to implement the community-level NPIs that are more disruptive to society (e.g., temporary K–12 school closures) during pandemics of moderate severity. In each locality, the goal should be to implement NPIs early enough and long enough to maximize effectiveness while minimizing economic and social costs to ensure that NPIs are commensurate to the pandemic severity.
New Elements Added in 2017
New elements in this report, in addition to the evidence-based NPI recommendations, include a summary of key lessons learned from the 2009 H1N1 pandemic response (Box 1), information on community engagement and preparedness (supplementary Chapter 1 https://stacks.cdc.gov/view/cdc/44313), and information on new or updated pandemic assessment tools (supplementary Chapter 2 https://stacks.cdc.gov/view/cdc/44313), which include the novel influenza virus pandemic intervals tool, the Influenza Risk Assessment Tool, and PSAF. As described in the following sections, this report also presents two additional planning tools designed to assist states and localities in ensuring pandemic preparedness.
Prepandemic Planning Scenarios for NPI Implementation According to Pandemic Severity
During the initial stages of a pandemic, CDC will use the PSAF tool to prepare an initial assessment of pandemic severity that provides early guidance on use of NPIs to help slow the transmission of the novel virus. To facilitate the use of the initial assessment information by state and local health departments, CDC has provided a set of four prepandemic planning scenarios. Each scenario aligns with one of the four assessment quadrants (Figure 3) and provides information on past influenza pandemics for comparison (Table 9). These planning scenarios are designed to facilitate state and local prepandemic planning for NPI implementation according to pandemic severity (as classified by PSAF) ( Figure 6) (Tables 9 and 10). After sufficient epidemiologic data are accrued and the refined assessment of pandemic severity becomes available, CDC will issue updated pandemic NPI guidance, which will be tailored more precisely to the specific pandemic. Additional information about the planning scenarios and phasing of NPIs is available (supplementary Chapter 2 https://stacks.cdc.gov/view/cdc/44313).
Supplemental Prepandemic NPI Planning Guides
The 2007 report included supplemental prepandemic NPI planning guides for individuals and families; child care programs, K–12 schools, and institutions of higher education; community- and faith-based organizations; and businesses and other workplaces. These guides have been updated, and two new guides have been developed for public health communicators and event planners that address NPI communications and modification, postponement, or cancellation of mass gatherings. These guides are intended to help operationalize the 2017 update and provide specific information that can assist different groups in their prepandemic planning and decision-making (https://www.cdc.gov/nonpharmaceutical-interventions).
Future Research
Although progress has been made since 2009 toward building the evidence base for use of NPIs to slow the spread of pandemic influenza, additional research is needed. For personal NPIs, areas for additional research include evaluating the effects of increased frequency and quality of hand washing on influenza virus transmission, determining the role of infected persons who are not symptomatic in the transmission of influenza viruses in households, and assessing the effectiveness, acceptability, and feasibility of recommending face mask use by well persons in community settings as a means of avoiding infection during a pandemic. For community NPIs, one topic for additional study involves gathering empirical data on social mixing patterns in schools and community settings. These data can be used to create high-fidelity, high-resolution mathematical models of virus transmission in these settings to facilitate data-driven evaluations of different social distancing measures. Another area of research for community NPIs involves assessing the potential secondary consequences (e.g., missed work) of select community-level measures (e.g., school closures) for families, communities, and society to assess the economic effects of these measures. For environmental NPIs, additional research is needed to better understand surface contamination (e.g., which types of surfaces are more likely to be contaminated with influenza viruses) and identify situations in which surface cleaning should be emphasized (e.g., in households with confirmed influenza cases versus in healthy households). Additional information about NPI research gaps is available (supplementary Chapter 3 https://stacks.cdc.gov/view/cdc/44313).
Conclusion
The 2009 H1N1 pandemic provided an opportunity to test, in practice, the key concepts of NPIs in mitigating the impact of an influenza pandemic, just 2 years after the publication of the 2007 guidance. As the experience from 2009 has shown, NPIs can be a critical component of pandemic influenza mitigation. Although well-matched pandemic vaccines remain the main tool in reducing the risk of acquiring infection and in controlling the spread of a pandemic virus, vaccines might not be widely available for up to 6 months after the emergence of a pandemic influenza virus, given current vaccine production technology. Furthermore, as during the 2009 H1N1 pandemic, antiviral medications might be prioritized for treatment but not used for widespread chemoprophylaxis because of concerns about antiviral resistance and limited stockpiles of antiviral medications. Therefore, NPIs might be the only prevention tools readily available for persons and communities to help slow transmission of an influenza virus during the initial stages of a pandemic. However, individual NPIs might be only partially effective in limiting community transmission when implemented alone. Thus, the most efficient implementation involves early, targeted, and layered use of multiple NPIs (https://www.cdc.gov/flu/pandemic-resources/planning-preparedness/community-mitigation.html). In addition, some community-level NPIs that potentially have the greatest epidemiologic effects on pandemic influenza virus transmission in communities, most notably school closures and dismissals, also are most likely to be associated with secondary (unwanted) consequences (104). Hence, prepandemic planning, including engaging communities in planning activities well ahead of the next pandemic, is critical to enable appropriate local decision-making during the early stages of a pandemic.
After the 2009 H1N1 pandemic, evidence on the effectiveness and feasibility of NPIs expanded substantially. A summary of the evidence in this 2017 update includes 2009 H1N1-related research (supplementary Appendix 5 https://stacks.cdc.gov/view/cdc/44314). However, knowledge gaps remain and should be addressed by future research. Further updates of these guidelines will be developed and issued when significant new information and evidence emerges about the effectiveness and feasibility of NPIs in mitigating the impact of pandemic influenza.
CDC Community Mitigation Guidelines Work Group
Alexandra Levitt, PhD, Office of Infectious Diseases, CDC,; Stephanie Dopson, ScD, Influenza Coordination Unit, Office of Infectious Diseases, CDC; Mark Frank, MPH, Influenza Coordination Unit, Office of Infectious Diseases, CDC; Rachel Holloway, Influenza Coordination Unit, Office of Infectious Diseases, CDC; Lisa Koonin, DrPH, Influenza Coordination Unit, Office of Infectious Diseases, CDC; Sonja Rasmussen, MD, Influenza Coordination Unit, Office of Infectious Diseases, CDC; Stephen Redd, MD, Influenza Coordination Unit, Office of Infectious Diseases, CDC; Christopher de la Motte Hurst, MPH, Division of Global Migration and Quarantine, National Center for Emerging and Zoonotic Infectious Diseases, CDC; Neha Kanade, MPH, Division of Global Migration and Quarantine, National Center for Emerging and Zoonotic Infectious Diseases, CDC; Noreen Qualls, DrPH, Division of Global Migration and Quarantine, National Center for Emerging and Zoonotic Infectious Diseases, CDC; Jeanette Rainey, PhD, Division of Global Migration and Quarantine, National Center for Emerging and Zoonotic Infectious Diseases, CDC; Amra Uzicanin, MD, Division of Global Migration and Quarantine, National Center for Emerging and Zoonotic Infectious Diseases, CDC; Matthew Biggerstaff, MPH, Influenza Division, National Center for Immunization and Respiratory Diseases, CDC; Daniel Jernigan, MD, Influenza Division, National Center for Immunization and Respiratory Diseases, CDC; Carrie Reed, DSc, Influenza Division, National Center for Immunization and Respiratory Diseases, CDC.CDC Community Mitigation Guidelines Teams
Coordination Team
Alexandra Levitt, PhD, Office of Infectious Diseases; Narue Wright-Jegede, MPH, Neha Kanade, MPH, and Noreen Qualls, DrPH, Division of Global Migration and Quarantine, National Center for Emerging and Zoonotic Infectious Diseases, CDC.
Abstraction Team
Yao-Hsuan Chen, PhD, Charissa Dowdye, MPH, Hongjiang Gao, PhD, Narue Wright-Jegede, MPH, Neha Kanade, MPH, Jasmine Kenney, MPH, Erin Keyes, MPH, Tiffani Phelps, MPH, Noreen Qualls, DrPH, Jeanette Rainey, PhD, Jianrong Shi, MD, Karen Wong, MD, and Yenlik Zheteyeva, MD, Community Interventions for Infection Control Unit, Division of Global Migration and Quarantine, National Center for Emerging and Zoonotic Infectious Diseases, CDC.
Consultation Team
Maleeka Glover, ScD, Influenza Coordination Unit, Office of Infectious Diseases; Rita Helfand, MD, Office of the Director, National Center for Emerging and Zoonotic Infectious Diseases; Clive Brown, FRSPH, Martin Cetron, MD, Pamela Diaz, MD, Katrin Kohl, MD, PhD, David McAdam, MPA, and Jessica Reichard, Division of Global Migration and Quarantine, National Center for Emerging and Zoonotic Infectious Diseases; Bryan Christensen, PhD, Carolyn Gould, MD, Jeff Hageman, MD, John Jernigan, MD, and David Kuhar, MD, Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases; William Potts-Datema, MS, and Mary Vernon-Smiley, MD, Division of Adolescent and School Health, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention; Belinda Smith and Teresa Smith, RN, Health Communication Science Office, National Center for Immunization and Respiratory Diseases; Carolyn Bridges, MD, and Samuel Graitcer, MD, Immunization Services Division, National Center for Immunization and Respiratory Diseases; Joseph Bresee, MD, Influenza Division, National Center for Immunization and Respiratory Diseases; Lisa Delaney, MD, and Chad Dowell, MD, Office of the Director, National Institute for Occupational Safety and Health; Samuel Groseclose, DVM, Laura Leidel, MSN, and Carol Pertowski, MD, Office of the Director, Office of Public Health Preparedness and Response; Steven Boedigheimer, MBA, Christa Singleton, MD, Theresa Smith, MD, and Todd Talbert, MA, Division of State and Local Readiness, Office of Public Health Preparedness and Response, CDC.
Conflict of Interest
The CDC contributors wish to disclose that they have no financial or competing interests or other relationships that would unfairly influence these CDC guidelines and recommendations.Corresponding author: Noreen Qualls, Division of Global Migration and Quarantine, National Center for Emerging and Zoonotic Infectious Diseases, CDC. Telephone: 404-639-8195; E-mail: nqualls@cdc.gov.
1Division of Global Migration and Quarantine, National Center for Emerging and Zoonotic Infectious Diseases, CDC, Atlanta, Georgia; 2Office of Infectious Diseases, CDC, Atlanta, Georgia; 3Eagle Medical Services, San Antonio, Texas; 4Karna, Atlanta, Georgia; 5Division of State and Local Readiness, Office of Public Health Preparedness and Response, CDC, Atlanta, Georgia; 6Influenza Division, National Center for Immunization and Respiratory Diseases, CDC, Atlanta, Georgia
* The updated 2017 planning guidelines do not address pandemic vaccine development and distribution, use of respirators in community or health care settings during a pandemic, or travel restrictions during a pandemic. Guidance and policies in these areas will be developed separately, as needed.
† In July 2009, ACIP recommended vaccination of several population groups, including children aged 6 months through 18 years because cases of H1N1 influenza had occurred in children who were in close contact with each other in school and child care settings (https://www.cdc.gov/h1n1flu/vaccination/acip.htm).
§ The pandemic mitigation framework proposed in the 2007 strategy was based on the early, targeted, and layered use of multiple NPIs. NPIs should be initiated early in a pandemic before local epidemics grow exponentially, be targeted toward those at the nexus of transmission (in affected areas where the novel virus circulates), and be layered together to reduce community transmission as much as possible. A list of NPIs that are recommended at all times and those that are reserved for pandemics is provided (Table 1).
¶ Influenza pandemics can range from mild to extreme in terms of rates of viral transmission and clinical severity, as described in the four prepandemic planning scenarios developed by CDC. A very severe or extreme pandemic, such as the 1918 pandemic, is characterized by high to very high transmissibility and clinical severity. A severe pandemic, such as the pandemics of 1957 and 1968, is characterized by high transmissibility and low to medium clinical severity. A mild or moderate pandemic, such as the 2009 pandemic, is characterized by low to medium transmissibility and clinical severity.
** Although many authorities use either 100°F or 100.4°F (37.8°C) as indicative of fever, this number can vary depending on factors such as the method of measurement and the age of the person. Therefore, other values for fever could be appropriate. CDC has public health recommendations that are based on the presence (or absence) of fever (i.e., persons’ temperature is not higher than their own normal temperature).
†† Guidance for caring for persons at home who have influenza symptoms is available at https://www.cdc.gov/flu/consumer/caring-for-someone.htm.
§§ Additional information about isolation and quarantine is available at https://www.cdc.gov/quarantine/QuarantineIsolation.html.
¶¶ If the incubation period for the next pandemic were shorter or longer than 3 days, CDC would amend the recommendation accordingly.
*** Additional information on the use of preemptive, coordinated school closures and dismissals of different durations is available (supplementary Chapter 3 https://stacks.cdc.gov/view/cdc/44313).
††† Persons at high risk for influenza-related complications include children aged <5 years (and especially aged <2 years), adults aged ≥65 years, pregnant women, residents of nursing homes and other long-term care facilities, and American Indians/Alaska Natives. Those at high risk also include persons with asthma, neurological and neurodevelopmental conditions, chronic lung disease, and heart disease; disorders of the blood, endocrine system, kidney, or liver; metabolic disorders; and weakened immune systems from disease or medication. Two other groups at higher risk are persons aged <19 years who receive long-term aspirin therapy and those with extreme obesity (https://www.cdc.gov/flu/about/disease/high_risk.htm).
§§§ As of September 2016, the U.S. workforce included 62.9% of the civilian, noninstitutionalized population aged ≥16 years (Source: US Bureau of Labor Statistics. The employment situation—September 2016. Washington, DC: US Department of Labor; 2016. https://www.bls.gov/news.release/archives/empsit_10072016.pdf).
¶¶¶ Antimicrobial products registered for use against H1N1 influenza and other influenza A viruses on hard surfaces (https://archive.epa.gov/pesticides/oppad001/web/pdf/influenza-a-product-list.pdf).
References
- Barrios LC, Koonin LM, Kohl KS, Cetron M. Selecting nonpharmaceutical strategies to minimize influenza spread: the 2009 influenza A (H1N1) pandemic and beyond. Public Health Rep 2012;127:565–71. CrossRef PubMed
- Monto AS, Webster RG. [Chapter 2]. In: Webster RG, Monto AS, Braciale TJ, Lamb RA, eds. Textbook of influenza. 2nd. West Sussex, UK: John Wiley and Sons, Ltd; 2013:20–33.
- Shrestha SS, Swerdlow DL, Borse RH, et al. Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009–April 2010). Clin Infect Dis 2011;52(Suppl 1):S75–82.CrossRef PubMed
- Jhung MA, Swerdlow D, Olsen SJ, et al. Epidemiology of 2009 pandemic influenza A (H1N1) in the United States. Clin Infect Dis 2011;52(Suppl 1):S13–26. CrossRef PubMed
- CDC. Updated CDC estimates of 2009 H1N1 influenza cases, hospitalizations, and deaths in the United States, April 2009–April 10, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010. https://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm
- Labrosse B, Tourdjman M, Porcher R, et al. Detection of extensive cross-neutralization between pandemic and seasonal A/H1N1 influenza viruses using a pseudotype neutralization assay. PLoS One 2010;5:e11036. CrossRef PubMed
- Nougairède A, Ninove L, Zandotti C, et al. Novel virus influenza A (H1N1sw) in South-Eastern France, April–August 2009. PLoS One 2010;5:e9214. CrossRef PubMed
- CDC. Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009;58(No. RR-10). PubMed
- Fell DB, Sprague AE, Liu N, et al. Better Outcomes Registry & Network (BORN) Ontario. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health 2012;102:e33–40. CrossRef PubMed
- Richards JL, Hansen C, Bredfeldt C, et al. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clin Infect Dis 2013;56:1216–22. CrossRef PubMed
- CDC. Update: novel influenza A (H1N1) virus infection—Mexico, March–May, 2009. MMWR Morb Mortal Wkly Rep 2009;58:585–9. PubMed
- CDC. CDC guidance for state and local public health officials and school administrators for school (K–12) responses to influenza during the 2009–2010 school year. Atlanta, GA: US Department of Health and Human Services, CDC; 2010. https://www.cdc.gov/h1n1flu/schools/schoolguidance.htm
- CDC. Swine influenza A (H1N1) infection in two children—Southern California, March–April 2009. MMWR Morb Mortal Wkly Rep 2009;58:400–2. PubMed
- CDC. Telebriefing on investigation of human cases of H1N1 flu. Atlanta, GA: US Department of Health and Human Services, CDC. May 5, 2009. https://www.cdc.gov/media/transcripts/2009/t090505.htm
- Kann L, Kinchen S, Modzelski B, et al. ILI-related school dismissal monitoring system: an overview and assessment. Disaster Med Public Health Prep 2012;6:104–12. CrossRef PubMed
- Talaat M, Afifi S, Dueger E, et al. Effects of hand hygiene campaigns on incidence of laboratory-confirmed influenza and absenteeism in schoolchildren, Cairo, Egypt. Emerg Infect Dis 2011;17:619–25. CrossRef PubMed
- Chao DL, Halloran ME, Longini IM . School opening dates predict pandemic influenza A(H1N1) outbreaks in the United States. J Infect Dis 2010;202:877–80. CrossRef PubMed
- Copeland DL, Basurto-Davila R, Chung W, et al. Effectiveness of a school district closure for pandemic influenza A (H1N1) on acute respiratory illnesses in the community: a natural experiment. Clin Infect Dis 2013;56:509–16. CrossRef PubMed
- Earn DJ, He D, Loeb MB, Fonseca K, Lee BE, Dushoff J. Effects of school closure on incidence of pandemic influenza in Alberta, Canada. Ann Intern Med 2012;156:173–81. CrossRefPubMed
- Chowell G, Echevarría-Zuno S, Viboud C, et al. Characterizing the epidemiology of the 2009 influenza A/H1N1 pandemic in Mexico. PLoS Med 2011;8:e1000436. CrossRef PubMed
- Community Preventive Services Task Force. Emergency preparedness: school dismissals to reduce transmission of pandemic influenza [Internet]. Guide to Community Preventive Services (The Community Guide); US Department of Health and Human Services, CDC; 2012. https://www.thecommunityguide.org/findings/emergency-preparedness-and-response-school-dismissals-reduce-transmission-pandemic-influenza
- Herrera-Valdez MA, Cruz-Aponte M, Castillo-Chavez C. Multiple outbreaks for the same pandemic: local transportation and social distancing explain the different “waves” of A-H1N1pdm cases observed in México during 2009. Math Biosci Eng 2011;8:21–48. CrossRef PubMed
- Nasrullah M, Breiding MJ, Smith W, et al. Response to 2009 pandemic influenza A H1N1 among public schools of Georgia, United States—fall 2009. Int J Infect Dis 2012;16:e382–90.CrossRef PubMed
- Kimberlin DW, Escude J, Gantner J, et al. Targeted antiviral prophylaxis with oseltamivir in a summer camp setting. Arch Pediatr Adolesc Med 2010;164:323–7. CrossRef PubMed
- Ward KA, Armstrong P, McAnulty JM, Iwasenko JM, Dwyer DE. Outbreaks of pandemic (H1N1) 2009 and seasonal influenza A (H3N2) on cruise ship. Emerg Infect Dis 2010;16:1731–7.CrossRef PubMed
- Pebody RG, Harris R, Kafatos G, et al. Use of antiviral drugs to reduce household transmission of pandemic (H1N1) 2009, United Kingdom. Emerg Infect Dis 2011;17:990–9. CrossRefPubMed
- Harvard TH Chan School of Public Health. Harvard Opinion Research Program [Internet]. Public response to H1N1. Boston, MA: Harvard TH Chan School of Public Health. https://www.hsph.harvard.edu/horp/project-on-the-public-response-to-h1n1
- CDC. Parental attitudes and experiences during school dismissals related to 2009 influenza A (H1N1)—United States, 2009. MMWR Morb Mortal Wkly Rep 2010;59:1131–4. PubMed
- Agolory SG, Barbot O, Averhoff F, et al. Implementation of non-pharmaceutical interventions by New York City public schools to prevent 2009 influenza A. PLoS One 2013;8:e50916.CrossRef PubMed
- Klaiman T, Kraemer JD, Stoto MA. Variability in school closure decisions in response to 2009 H1N1: a qualitative systems improvement analysis. BMC Public Health 2011;11:73. CrossRefPubMed
- Enanoria WT, Crawley AW, Tseng W, Furnish J, Balido J, Aragón TJ. The epidemiology and surveillance response to pandemic influenza A (H1N1) among local health departments in the San Francisco Bay Area. BMC Public Health 2013;13:276. CrossRef PubMed
- Drago R, Miller K. Sick at work: infected employees in the workplace during the H1N1 pandemic. Institute for Women’s Policy Research; 2010: No. B264.
- Potter MA, Brown ST, Cooley PC, et al. School closure as an influenza mitigation strategy: how variations in legal authority and plan criteria can alter the impact. BMC Public Health 2012;12:977. CrossRef PubMed
- Bell DM, Weisfuse IB, Hernandez-Avila M, Del Rio C, Bustamante X, Rodier G. Pandemic influenza as 21st century urban public health crisis. Emerg Infect Dis 2009;15:1963–9. CrossRefPubMed
- SteelFisher GK. Blendon RJ, Kang M, et al. Adoption of preventive behaviors in response to the 2009 H1N1 influenza pandemic: a multiethnic perspective. Influenza Other Respir Viruses 2015;9:131–42.
- Quinn SC, Parmer J, Freimuth VS, Hilyard KM, Musa D, Kim KH. Exploring communication, trust in government, and vaccination intention later in the 2009 H1N1 pandemic: results of a national survey. Biosecur Bioterror 2013;11:96–106. CrossRef PubMed
- Peiris JSM, Yu WC, Leung CW, et al. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 2004;363:617–9. CrossRef PubMed
- Gift TL, Palekar RS, Sodha SV, et al. ; Pennsylvania H1N1 Working Group. Household effects of school closure during pandemic (H1N1) 2009, Pennsylvania, USA. Emerg Infect Dis 2010;16:1315–7. CrossRef PubMed
- Borse RH, Behravesh CB, Dumanovsky T, et al. Closing schools in response to the 2009 pandemic influenza A H1N1 virus in New York City: economic impact on households. Clin Infect Dis 2011;52(Suppl 1):S168–72. CrossRef PubMed
- Chen WC, Huang AS, Chuang JH, Chiu CC, Kuo HS. Social and economic impact of school closure resulting from pandemic influenza A/H1N1. J Infect 2011;62:200–3. CrossRef PubMed
- Mizumoto K, Yamamoto T, Nishiura H. Contact behaviour of children and parental employment behaviour during school closures against the pandemic influenza A (H1N1-2009) in Japan. J Int Med Res 2013;41:716–24. CrossRef PubMed
- Lau CH, Springston EE, Sohn MW, et al. Hand hygiene instruction decreases illness-related absenteeism in elementary schools: a prospective cohort study. BMC Pediatr 2012;12:52.CrossRef PubMed
- Holloway R, Rasmussen SA, Zaza S, Cox NJ, Jernigan DB. Updated preparedness and response framework for influenza pandemics. MMWR Recomm Rep 2014;63(No. RR-6). PubMed
- Reed C, Biggerstaff M, Finelli L, et al. Novel framework for assessing epidemiologic effects of influenza epidemics and pandemics. Emerg Infect Dis 2013;19:85–91. CrossRef PubMed
- Bell D, Nicoll A, Fukuda K, et al. World Health Organization Writing Group. Non-pharmaceutical interventions for pandemic influenza, national and community measures. Emerg Infect Dis 2006;12:88–94. PubMed
- Morse SS, Garwin RL, Olsiewski PJ. Public health. Next flu pandemic: what to do until the vaccine arrives? Science 2006;314:929. CrossRef PubMed
- Hatchett RJ, Mecher CE, Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc Natl Acad Sci U S A 2007;104:7582–7. CrossRefPubMed
- Markel H, Lipman HB, Navarro JA, et al. Nonpharmaceutical interventions implemented by US cities during the 1918-1919 influenza pandemic. JAMA 2007;298:644–54. CrossRefPubMed
- Institute of Medicine (US). Modeling community containment for pandemic influenza: a letter report. Washington, DC: The National Academies Press; 2006.
- Wu JT, Riley S, Fraser C, Leung GM. Reducing the impact of the next influenza pandemic using household-based public health interventions. PLoS Med 2006;3:e361. CrossRef PubMed
- Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature 2006;442:448–52. CrossRef PubMed
- Institute of Medicine (US) Forum on Microbial Threats. The domestic and international impacts of the 2009-H1N1 influenza A pandemic. Global challenges, global solutions: workshop summary. Washington, DC: The National Academies Press; 2010.
- Halloran ME, Ferguson NM, Eubank S, et al. Modeling targeted layered containment of an influenza pandemic in the United States. Proc Natl Acad Sci U S A 2008;105:4639–44. CrossRefPubMed
- Collignon PJ, Carnie JA. Infection control and pandemic influenza. Med J Aust 2006;185(Suppl):S54–7. PubMed
- Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis 2007;7:257–65. CrossRef PubMed
- Hall CB, Douglas RG . Nosocomial influenza infection as a cause of intercurrent fevers in infants. Pediatrics 1975;55:673–7. PubMed
- Memoli MJ, Athota R, Reed S, et al. The natural history of influenza infection in the severely immunocompromised vs nonimmunocompromised hosts. Clin Infect Dis 2014;58:214–24.CrossRef PubMed
- Levy JW, Suntarattiwong P, Simmerman JM, et al. Increased hand washing reduces influenza virus surface contamination in Bangkok households, 2009–2010. Influenza Other Respir Viruses 2014;8:13–6.
- Bean B, Moore BM, Sterner B, Peterson LR, Gerding DN, Balfour HH . Survival of influenza viruses on environmental surfaces. J Infect Dis 1982;146:47–51. CrossRef PubMed
- Thomas Y, Boquete-Suter P, Koch D, Pittet D, Kaiser L. Survival of influenza virus on human fingers. Clin Microbiol Infect 2014;20:O58–64. CrossRef PubMed
- Cori A, Valleron AJ, Carrat F, Scalia Tomba G, Thomas G, Boëlle PY. Estimating influenza latency and infectious period durations using viral excretion data. Epidemics 2012;4:132–8. CrossRefPubMed
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the U.S.: measuring disease burden and costs. Vaccine 2007;25:5086–96. CrossRefPubMed
- Sato M, Hosoya M, Kato K, Suzuki H. Viral shedding in children with influenza virus infections treated with neuraminidase inhibitors. Pediatr Infect Dis J 2005;24:931–2. CrossRefPubMed
- Hall CB, Douglas RG , Geiman JM, Meagher MP. Viral shedding patterns of children with influenza B infection. J Infect Dis 1979;140:610–3. CrossRef PubMed
- Neuzil KM, Hohlbein C, Zhu Y. Illness among schoolchildren during influenza season: effect on school absenteeism, parental absenteeism from work, and secondary illness in families. Arch Pediatr Adolesc Med 2002;156:986–91. CrossRef PubMed
- Glezen WP, Couch RB, MacLean RA, et al. Interpandemic influenza in the Houston area, 1974–76. N Engl J Med 1978;298:587–92. CrossRef PubMed
- Fox JP, Cooney MK, Hall CE, Foy HM. Influenzavirus infections in Seattle families, 1975–1979. II. Pattern of infection in invaded households and relation of age and prior antibody to occurrence of infection and related illness. Am J Epidemiol 1982;116:228–42. CrossRef PubMed
- Guclu H, Read J, Vukotich CJ , et al. Social contact networks and mixing among students in K–12 schools in Pittsburgh, PA. PLoS One 2016;11:e0151139. CrossRef PubMed
- Leecaster M, Toth DJ, Pettey WB, et al. Estimates of social contact in a middle school based on self-report and wireless sensor data. PLoS One 2016;11:e0153690. CrossRef PubMed
- Hens N, Ayele GM, Goeyvaerts N, et al. Estimating the impact of school closure on social mixing behaviour and the transmission of close contact infections in eight European countries. BMC Infect Dis 2009;9:187. CrossRef PubMed
- Eames KT, Tilston NL, White PJ, Adams E, Edmunds WJ. The impact of illness and the impact of school closure on social contact patterns. Health Technol Assess 2010;14:267–312. CrossRefPubMed
- Nichol KL, Lind A, Margolis KL, et al. The effectiveness of vaccination against influenza in healthy, working adults. N Engl J Med 1995;333:889–93. CrossRef PubMed
- Monto AS, Davenport FM, Napier JA, Francis T . Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. J Infect Dis 1970;122:16–25. CrossRefPubMed
- Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med 2001;344:889–96. CrossRefPubMed
- Mogabgab WJ. Acute respiratory illnesses in university (1962–1966), military and industrial (1962–1963) populations. Am Rev Respir Dis 1968;98:359–79. PubMed
- Layde PM, Engelberg AL, Dobbs HI, et al. Outbreak of influenza A/USSR/77 at Marquette University. J Infect Dis 1980;142:347–52. CrossRef PubMed
- Sobal J, Loveland FC. Infectious disease in a total institution: a study of the influenza epidemic of 1978 on a college campus. Public Health Rep 1982;97:66–72. PubMed
- Pons VG, Canter J, Dolin R. Influenza A/USSR/77 (H1N1) on a university campus. Am J Epidemiol 1980;111:23–30. CrossRef PubMed
- Nichol KL, Heilly SD, Ehlinger E. Colds and influenza-like illnesses in university students: impact on health, academic and work performance, and health care use. Clin Infect Dis 2005;40:1263–70. CrossRef PubMed
- Nichol KL, D’Heilly S, Ehlinger E. Burden of upper respiratory illnesses among college and university students: 2002-2003 and 2003-2004 cohorts. Vaccine 2006;24:6724–5. CrossRefPubMed
- Iuliano AD, Reed C, Guh A, et al. Notes from the field: outbreak of 2009 pandemic influenza A (H1N1) virus at a large public university in Delaware, April-May 2009. Clin Infect Dis 2009;49:1811–20. CrossRef PubMed
- Wang L, Chu C, Yang G, et al. Transmission characteristics of different students during a school outbreak of (H1N1) pdm09 influenza in China, 2009. Sci Rep 2014;4:5982. PubMed
- Glass RI, Brann EA, Slade JD, et al. Community-wide surveillance of influenza after outbreaks due to H3N2 (A/Victoria/75 and A/Texas/77) and H1N1 (A/USSR/77) influenza viruses, Mercer County, New Jersey, 1978. J Infect Dis 1978;138:703–6. CrossRef PubMed
- Nichol KL, D’Heilly S, Ehlinger EP. Influenza vaccination among college and university students: impact on influenzalike illness, health care use, and impaired school performance. Arch Pediatr Adolesc Med 2008;162:1113–8. CrossRef PubMed
- Wong KK, Shi J, Gao H, et al. Why is school closed today? Unplanned K–12 school closures in the United States, 2011–2013. PLoS One 2014;9:e113755. CrossRef PubMed
- Russell ES, Zheteyeva Y, Gao H, et al. Reactive school closure during increased influenza-like illness (ILI) activity in Western Kentucky, 2013: a field evaluation of effect on ILI incidence and economic and social consequences for families. Open Forum Infect Dis 2016;3:ofw113.
- Davis BM, Markel H, Navarro A, Wells E, Monto AS, Aiello AE. The effect of reactive school closure on community influenza-like illness counts in the state of Michigan during the 2009 H1N1 pandemic. Clin Infect Dis 2015;60:e90–7. CrossRef PubMed
- Gatwood J, Meltzer MI, Messonnier M, Ortega-Sanchez IR, Balkrishnan R, Prosser LA. Seasonal influenza vaccination of healthy working-age adults: a review of economic evaluations. Drugs 2012;72:35–48. CrossRef PubMed
- Balkhy HH, Memish ZA, Bafaqeer S, Almuneef MA. Influenza a common viral infection among Hajj pilgrims: time for routine surveillance and vaccination. J Travel Med 2004;11:82–6.CrossRef PubMed
- Abubakar I, Gautret P, Brunette GW, et al. Global perspectives for prevention of infectious diseases associated with mass gatherings. Lancet Infect Dis 2012;12:66–74. CrossRef PubMed
- Gutiérrez I, Litzroth A, Hammadi S, et al. Community transmission of influenza A (H1N1)v virus at a rock festival in Belgium, 2-5 July 2009. Euro Surveill 2009;14:19294. PubMed
- Rashid H, Haworth E, Shafi S, Memish ZA, Booy R. Pandemic influenza: mass gatherings and mass infection. Lancet Infect Dis 2008;8:526–7. CrossRef PubMed
- Rainey JJ, Phelps T, Shi J. Mass gatherings and respiratory disease outbreaks in the United States—should we be worried? Results from a systematic literature review and analysis of the National Outbreak Reporting System. PLoS One 2016;11:e0160378. CrossRef PubMed
- Blyth CC, Foo H, van Hal SJ, et al. World Youth Day 2008 Influenza Study Group. Influenza outbreaks during World Youth Day 2008 mass gathering. Emerg Infect Dis 2010;16:809–15.CrossRef PubMed
- Shi P, Keskinocak P, Swann JL, Lee BY. The impact of mass gatherings and holiday traveling on the course of an influenza pandemic: a computational model. BMC Public Health 2010;10:778.CrossRef PubMed
- Benkouiten S, Charrel R, Belhouchat K, et al. Circulation of respiratory viruses among pilgrims during the 2012 Hajj pilgrimage. Clin Infect Dis 2013;57:992–1000. CrossRef PubMed
- Memish ZA, Assiri A, Turkestani A, et al. Mass gathering and globalization of respiratory pathogens during the 2013 Hajj. Clin Microbiol Infect 2015;21:571.e1–8. CrossRef PubMed
- Bischoff WE, Swett K, Leng I, Peters TR. Exposure to influenza virus aerosols during routine patient care. J Infect Dis 2013;207:1037–46. CrossRef PubMed
- Weber TP, Stilianakis NI. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J Infect 2008;57:361–73. CrossRef PubMed
- Langmuir AD, Pizzi M, Trotter WY, Dunn FL. [Asian influenza surveillance]. Public Health Rep 1958;73:114–20. CrossRef PubMed
- Lim HC, Cutter J, Lim WK, Ee A, Wong YC, Tay BK. The influenza A (H1N1-2009) experience at the inaugural Asian Youth Games Singapore 2009: mass gathering during a developing pandemic. Br J Sports Med 2010;44:528–32. CrossRef PubMed
- Kelso JK, Milne GJ, Kelly H. Simulation suggests that rapid activation of social distancing can arrest epidemic development due to a novel strain of influenza. BMC Public Health 2009;9:117.CrossRef PubMed
- Sadique MZ, Edmunds WJ, Smith RD, et al. Precautionary behavior in response to perceived threat of pandemic influenza. Emerg Infect Dis 2007;13:1307–13. CrossRef PubMed
- Berkman BE. Mitigating pandemic influenza: the ethics of implementing a school closure policy. J Public Health Manag Pract 2008;14:372–8. CrossRef PubMed
 FIGURE 1. Goals of community mitigation for pandemic influenza
FIGURE 1. Goals of community mitigation for pandemic influenza
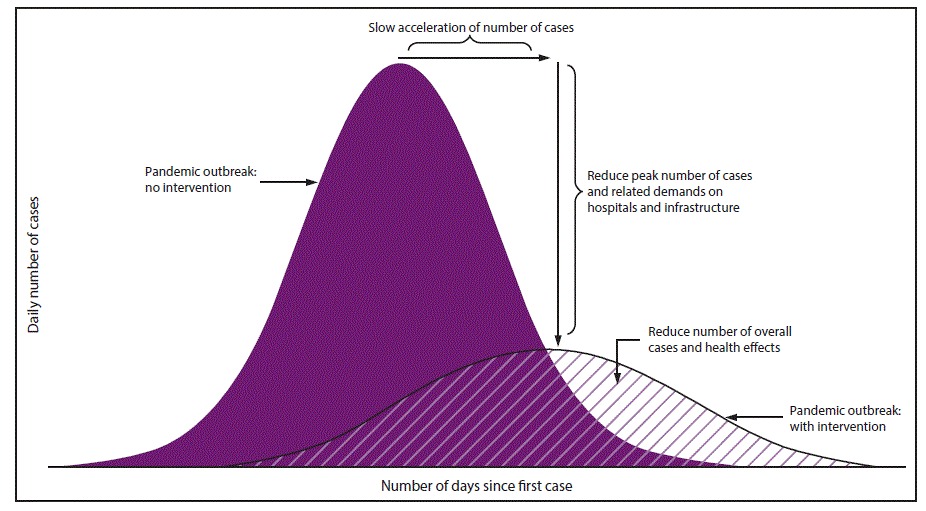
Source: Adapted from: CDC. Interim pre-pandemic planning guidance: community strategy for pandemic influenza mitigation in the United States—early, targeted, layered use of nonpharmaceutical interventions. Atlanta, GA: US Department of Health and Human Services, CDC; 2007. https://stacks.cdc.gov/view/cdc/11425.






















.png)










No hay comentarios:
Publicar un comentario